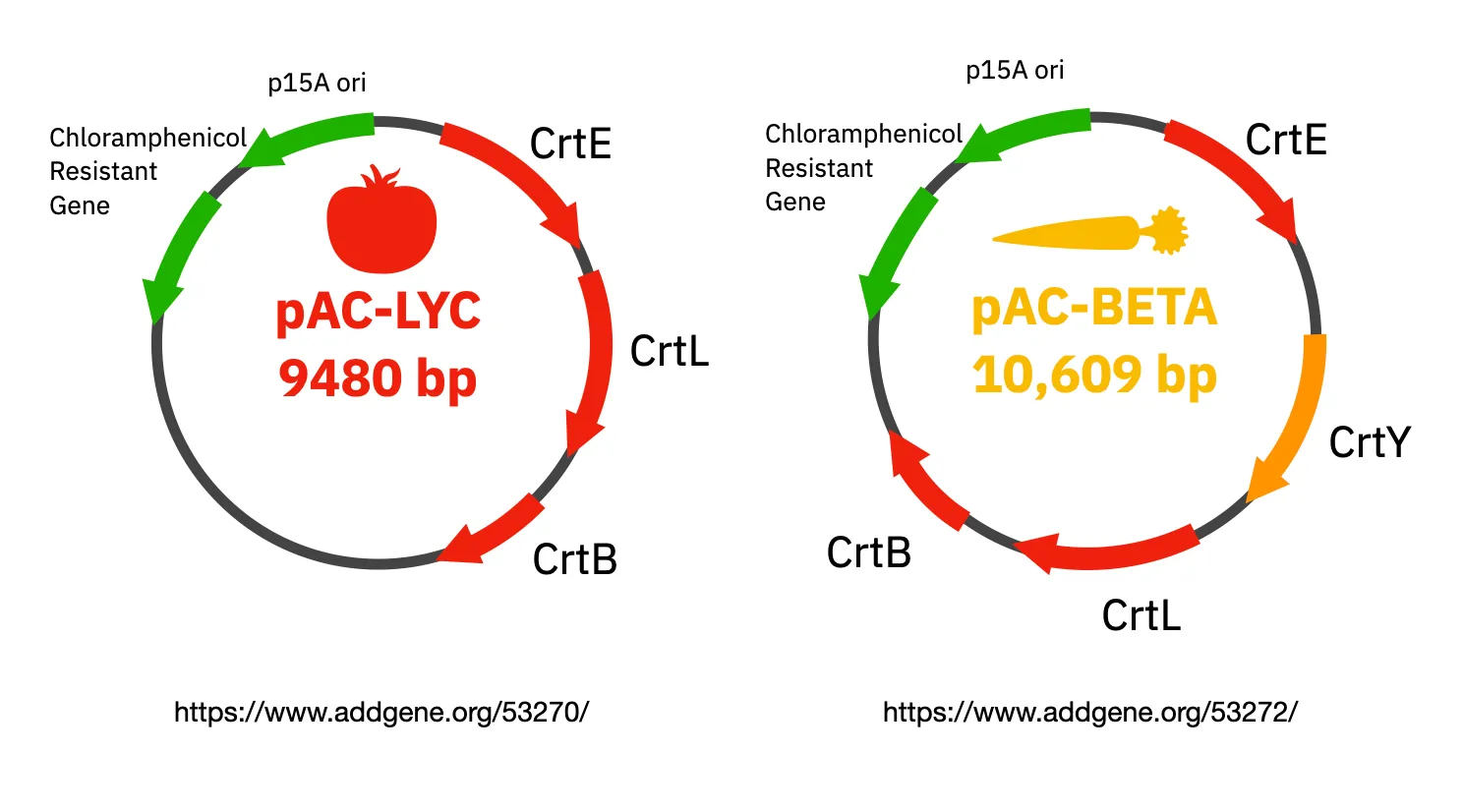
HTGAA 2025

Tip
Welcome to How to Grow (Almost) Anything — 2025. For full schedule, rooms, and policies, see [Logistics].
Get started
Class snapshot
- Lecture: Tuesdays, 2–5 PM ET — MIT E15‑341 & Zoom
- Recitation: Wednesdays, 5–6 PM ET — MIT E15‑359 & Zoom
- Lab: MIT 68‑083; see weekly pages for specifics
- Global Nodes: local recitations over Zoom
Details (Zoom links, exceptions, room notes) live on [Logistics].
Explore the course
- Class & recitation times, lab room, office hours, and global node note (2025).
- Lecture → Homework → Recitation → Lab → Reading & Resources.
- A three-part fast start for HTGAA 2025
- Specs, timelines, and deliverables — Due May 13, 2025
- Student pages grouped by node
What you’ll see each week
Big‑picture concepts, demos, and guest speakers. Slides and recordings are posted after each class.
Hands‑on assignments and documentation prompts. Clear checklists and notices help you get it done.
Interactive review and Q&A. Often includes short live demos or walkthroughs.
Wet‑lab or automation activities; protocols and inventory links included when relevant.
Curated references, tools, and papers to support the week’s topic.
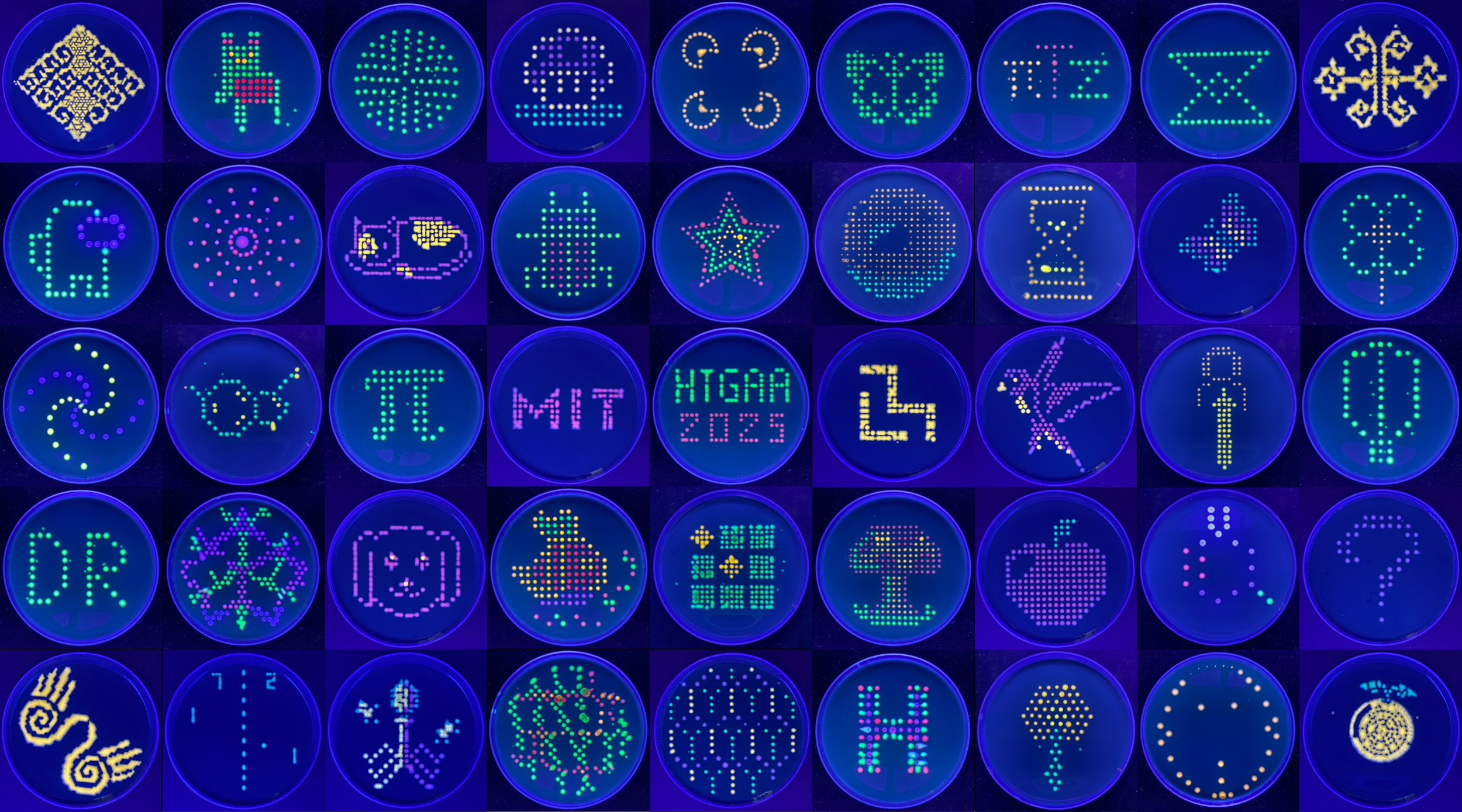
Gallery